The primary focus of this guidance is on adaptive designs for clinical trials intended to support the effectiveness and safety of drugs. FDA issues guidance for industry on adaptive designs for clinical trials of drugs and biologics. Adaptive design clinical trials for drugs and biologics.
Adaptive Design Clinical Trials For Drugs And Biologics, Adaptive design as defined by the US. The guidance describes the basic principles for designing conducting and reporting the results from an adaptive clinical trial and provides guidance to sponsors and applicants submitting investigational new drug applications INDs new drug applications NDAs biologics license applications BLAs or supplemental applications on the appropriate use of adaptive. Adaptive designs are applicable to both exploratory and confirmatory clinical trials.
 An Overview Of Platform Trials With A Checklist For Clinical Readers Sciencedirect From sciencedirect.com
An Overview Of Platform Trials With A Checklist For Clinical Readers Sciencedirect From sciencedirect.com
The primary focus of this guidance is on adaptive designs for clinical trials intended to support the effectiveness and safety of drugs and biological products. FDA has published a draft guidance on Adaptive Design Clinical Trials for Drugs and Biologics which gives regulatory guidance on methodological issues in exploratory and confirmatory clinical trials planned with an adaptive design. What is the Adaptive Designs for Clinical Trials of Drugs and Biologics Guidance for Industry. On November 29 the Food and Drug Administration FDA issued a final guidance for industry entitled Adaptive Designs for Clinical Trials of Drugs and Biologics Adaptive design clinical trials allow for prospectively planned modifications to one or more aspects of the design based on accumulating data from subjects in the trial.
The clinical trial landscape has changed since 2010 regards the FDAs stance on.
The primary focus of this guidance is on adaptive designs for clinical trials intended to support the effectiveness and safety of drugs. This comment summarizes the discussion within the joint working group Adaptive Designs and Multiple. Adaptive designs for exploratory clinical trials deal mainly with. Adaptive design as defined by the US. The primary focus of this guidance is on adaptive designs for clinical trials intended to support the effectiveness and safety of drugs. This guidance finalizes the draft guidance entitled Adaptive Designs for Clinical Trials of Drugs and Biologics issued in October 2018.
Read another article:
 Source: credevo.com
Source: credevo.com
Clinical Trial Designs Basket Umbrella Platform Trial Designs Part Ii Credevo Articles For the purposes of this guidance an adaptive design is defined as a clinical trial design that 44 allows for prospectively planned modifications to one or more aspects of the design based on. This guidance will replace the 2010 draft guidance for industry Adaptive Design Clinical Trials for Drugs and Biologics. Adaptive Design Clinical Trials for Drugs and Biologics which defines adaptive designs as studies that include a prospectively planned opportunity for modification of one or more specified aspects of the study design and hypotheses based on analysis of data. Adaptive designs for exploratory clinical trials deal mainly with.
 Source: valueinhealthjournal.com
Source: valueinhealthjournal.com
A Review Of Clinical Trials With An Adaptive Design And Health Economic Analysis Value In Health Adaptive designs for exploratory clinical trials deal mainly with. This guidance finalizes the draft guidance entitled Adaptive Designs for Clinical Trials of Drugs and Biologics issued in October 2018. The guidance describes the basic principles for designing conducting and reporting the results from an adaptive clinical trial and provides guidance to sponsors and applicants submitting investigational new drug applications INDs new drug applications NDAs biologics license applications BLAs or supplemental applications on the appropriate use of adaptive. An adaptive design is defined as a design that allows modifications to the trial andor statistical procedures of the trial after its initiation without undermining its validity and integrity.
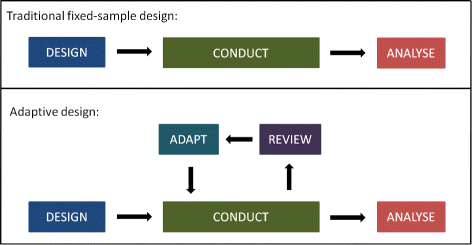 Source: bmcmedicine.biomedcentral.com
Source: bmcmedicine.biomedcentral.com
Adaptive Designs In Clinical Trials Why Use Them And How To Run And Report Them Bmc Medicine Full Text For the purposes of this guidance an adaptive design is defined as a clinical trial design that allows for prospectively planned modifications to one or more aspects of the design based on accumulating data from subjects in the trial. The 33-page guidance which finalizes a draft version released for comment in September 2018 and replaces an earlier guidance from 2010 sets out FDAs recommendations on adaptive trial design principles and the information FDA will review from adaptive studies submitted as part of investigational new drug applications INDs new drug applications. An adaptive design is defined as a design that allows modifications to the trial andor statistical procedures of the trial after its initiation without undermining its validity and integrity. For the purposes of this guidance an adaptive design is defined as a clinical trial design that allows for prospectively planned modifications to one or more aspects of the design based on accumulating data from subjects in the trial.
 Source: sciencedirect.com
Source: sciencedirect.com
Phase Ii Trials In Drug Development And Adaptive Trial Design Sciencedirect This comment summarizes the discussion within the joint working group Adaptive Designs and Multiple. In 2010 the US Food and Drug Administration FDA released a draft guidance Guidance for Industry. The clinical trial landscape has changed since 2010 regards the FDAs stance on. This comment summarizes the discussion within the joint working group Adaptive Designs and Multiple.
 Source: sciencedirect.com
Source: sciencedirect.com
An Overview Of Platform Trials With A Checklist For Clinical Readers Sciencedirect The September 2018 release replaces the 2010 draft guidance issued by the FDA. FDA has published a draft guidance on Adaptive Design Clinical Trials for Drugs and Biologics which gives regulatory guidance on methodological issues in exploratory and confirmatory clinical trials planned with an adaptive design. This comment summarizes the discussion within the joint working group Adaptive Designs and Multiple. Guidance for Industry Adaptive Design Clinical Trials for Drugs and Biologics Additional copies are available from.
 Source: iso.dzl100.com
Source: iso.dzl100.com
Adaptive Design Clinical Trials for Drugs and Biologics which defines adaptive designs as studies that include a prospectively planned opportunity for modification of one or more specified aspects of the study design and hypotheses based on analysis of data. Adaptive Design Clinical Trials for Drugs and Biologics Guidance for Industry December 2019 Download the Final Guidance Document Read the Federal Register Notice Final Level 1 Guidance. The guidance provides information to. In 2010 the US Food and Drug Administration FDA released a draft guidance Guidance for Industry.
 Source: acsjournals.onlinelibrary.wiley.com
Source: acsjournals.onlinelibrary.wiley.com
Clinical Trial Design Past Present And Future In The Context Of Big Data And Precision Medicine Li 2020 Cancer Wiley Online Library This guidance will replace the 2010 draft guidance for industry Adaptive Design Clinical Trials for Drugs and Biologics. For the purposes of this guidance an adaptive design is defined as a clinical trial design that 44 allows for prospectively planned modifications to one or more aspects of the design based on. The purpose is to. FDA has published a draft guidance on Adaptive Design Clinical Trials for Drugs and Biologics which gives regulatory guidance on methodological issues in exploratory and confirmatory clinical trials planned with an adaptive design.
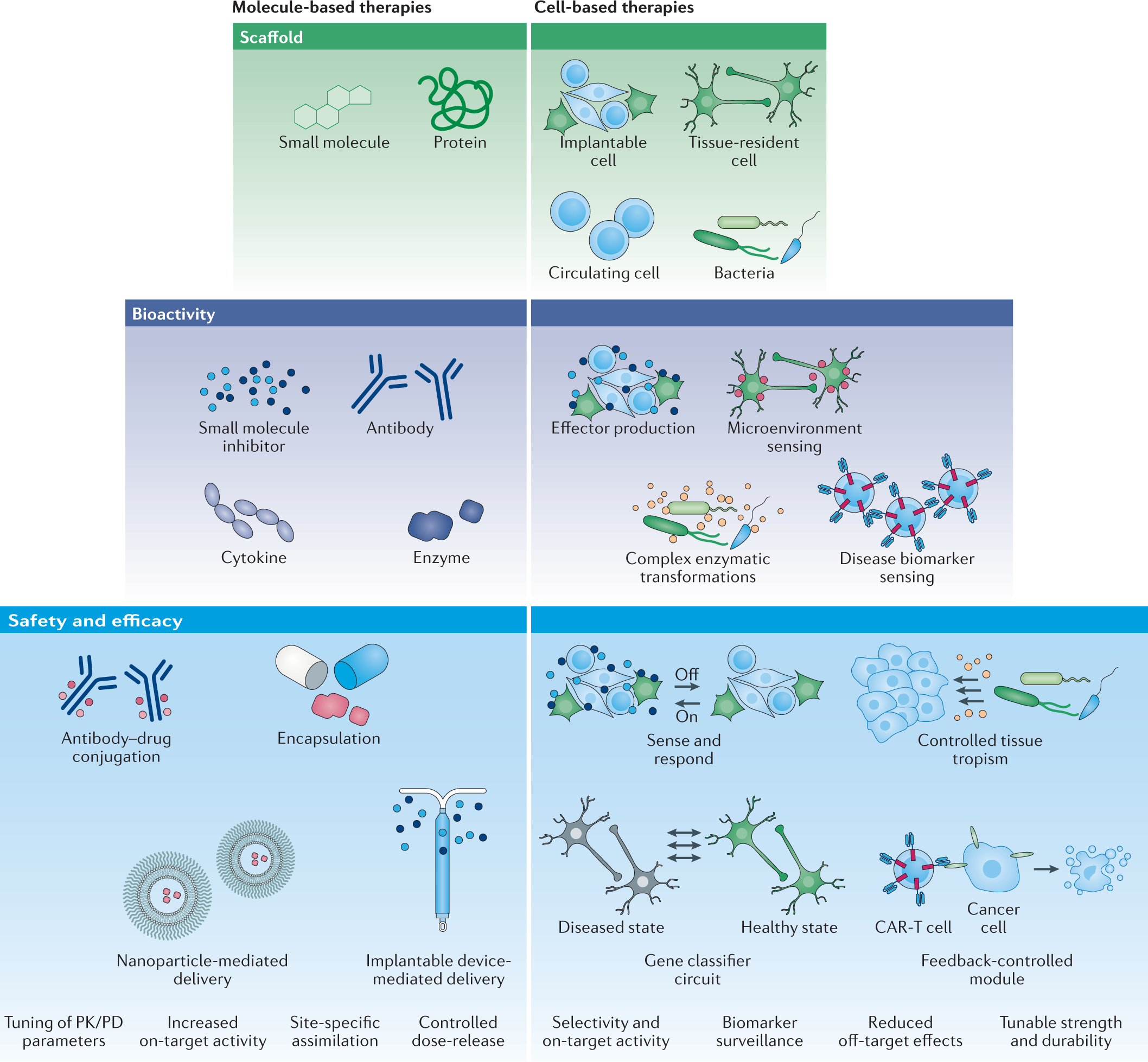 Source: nature.com
Source: nature.com
Engineering Living Therapeutics With Synthetic Biology Nature Reviews Drug Discovery Adaptive Design Clinical Trials for Drugs and Biologics which defines adaptive designs as studies that include a prospectively planned opportunity for modification of one or more specified aspects of the study design and hypotheses based on analysis of data. FDA is a clinical trial design that allows for prospectively planned modifications to one or more aspects of the design based on accumulating data from subjects in the trial 1 Adaptive design characteristics include modifying an ongoing clinical trial in accordance with predetermined rules based on data from interim analyses. Guidance for Industry Adaptive Design Clinical Trials for Drugs and Biologics Additional copies are available from. The primary focus of this guidance is on adaptive designs for clinical trials intended to support the effectiveness and safety of drugs.
 Source: ris.world
Source: ris.world
Usa Adaptive Designs For Clinical Trials Of Drugs And Biologics Ris World The clinical trial landscape has changed since 2010 regards the FDAs stance on. An adaptive design is defined as a design that allows modifications to the trial andor statistical procedures of the trial after its initiation without undermining its validity and integrity. The purpose is to. The US Food and Drug Administration FDA last week finalized guidance on adaptive clinical trial designs for drugs and biologicsThis document provides guidance to sponsors and applicants submitting investigational new drug applications INDs new drug applications NDAs biologics licensing applications BLAs or supplemental applications on.
 Source: quanticate.com
Source: quanticate.com
The What Why And How Of Adaptive Clinical Trials What is the Adaptive Designs for Clinical Trials of Drugs and Biologics Guidance for Industry. This guidance finalizes the draft guidance entitled Adaptive Designs for Clinical Trials of Drugs and Biologics issued in October 2018. An adaptive design is defined as a design that allows modifications to the trial andor statistical procedures of the trial after its initiation without undermining its validity and integrity. Guidance for Industry Adaptive Design Clinical Trials for Drugs and Biologics Additional copies are available from.
 Source: resmedjournal.com
Source: resmedjournal.com
In Line Treatments And Clinical Initiatives To Fight Against Covid 19 Outbreak Respiratory Medicine For the purposes of this guidance an adaptive design is defined as a clinical trial design that allows for prospectively planned modifications to one or more aspects of the design based on accumulating data from subjects in the trial. Adaptive designs are applicable to both exploratory and confirmatory clinical trials. The guidance describes important principles for designing conducting and reporting the results from an adaptive clinical trial. FDA is a clinical trial design that allows for prospectively planned modifications to one or more aspects of the design based on accumulating data from subjects in the trial 1 Adaptive design characteristics include modifying an ongoing clinical trial in accordance with predetermined rules based on data from interim analyses.
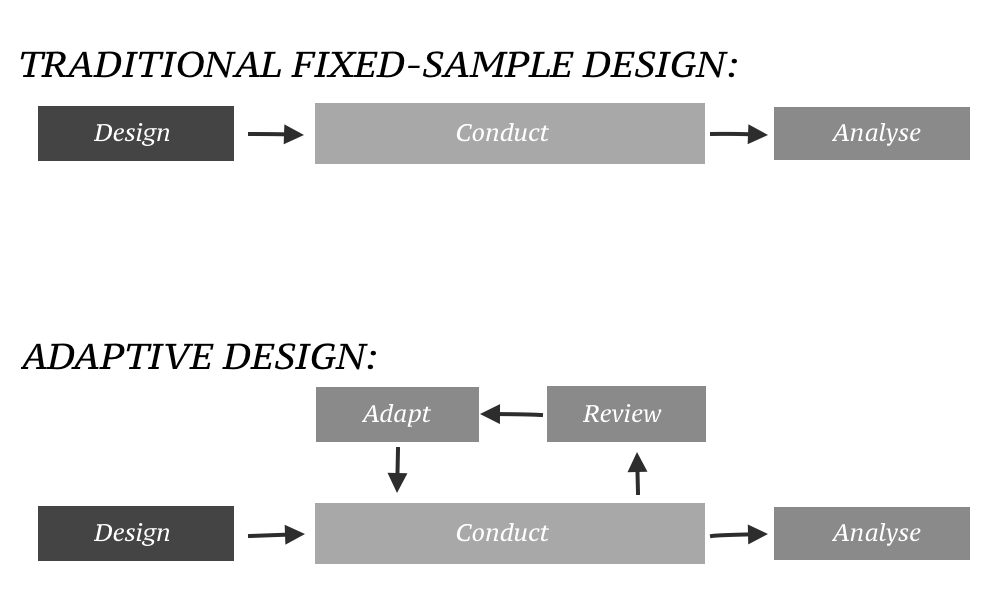 Source: en.wikipedia.org
Source: en.wikipedia.org
Adaptive Design Medicine Wikipedia An adaptive design is defined as a design that allows modifications to the trial andor statistical procedures of the trial after its initiation without undermining its validity and integrity. The September 2018 release replaces the 2010 draft guidance issued by the FDA. The primary focus of this guidance is on adaptive designs for clinical trials intended to support the effectiveness and safety of drugs and biological products. The guidance provides information to.
 Source: cell.com
Source: cell.com
Clinical Development Of Gene Therapies The First Three Decades And Counting Molecular Therapy Methods Clinical Development This guidance finalizes the draft guidance entitled Adaptive Designs for Clinical Trials of Drugs and Biologics issued in October 2018. Adaptive Design Clinical Trials for Drugs and Biologics Guidance for Industry December 2019 Download the Final Guidance Document Read the Federal Register Notice Final Level 1 Guidance. The primary focus of this guidance is on adaptive designs for clinical trials intended to support the effectiveness and safety of drugs and biological products. The purpose is to.
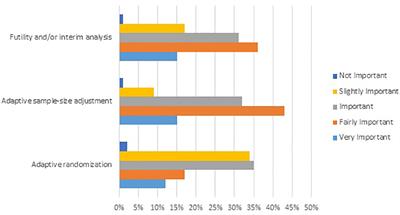 Source: frontiersin.org
Source: frontiersin.org
Frontiers Value Of Adaptive Trials And Surrogate Endpoints For Clinical Decision Making In Rare Cancers Oncology The guidance describes important principles for designing conducting and reporting the results from an adaptive clinical trial. This guidance will replace the 2010 draft guidance for industry Adaptive Design Clinical Trials for Drugs and Biologics. What is the Adaptive Designs for Clinical Trials of Drugs and Biologics Guidance for Industry. The clinical trial landscape has changed since 2010 regards the FDAs stance on.
 Source: nejm.org
Source: nejm.org
Newer Biologic And Small Molecule Therapies For Inflammatory Bowel Disease Nejm FDA issues guidance for industry on adaptive designs for clinical trials of drugs and biologics. In 2010 the US Food and Drug Administration FDA released a draft guidance Guidance for Industry. Adaptive design as defined by the US. By casting dose finding as a Bayesian model selection problem we propose an adaptive design by simultaneously incorporating the toxicity and efficacy outcomes to select the optimal biological dose OBD in phase III clinical trials.







